Micro-CT evaluation of the efficacy of hard-tissue removal from the root canal and isthmus area by positive and negative pressure irrigation systems
Abstract
Aim: To evaluate the removal of accumulated hard-tissue debris (AHTD) from the root canal system of mandibular molars by positive and negative pressure irrigation systems, using micro-CT imaging analysis.
Methodology: Mandibular molars with a single canal in the distal root and 2 canals connected by an isthmus in the mesial root were matched based on similar morphological dimensions using micro-CT evaluation and assigned to 2 experimental groups (n = 20 mesial and 10 distal canals), according to the irrigation protocol: apical positive (conventional irrigation) or negative (EndoVac system) pressure. Changes in root canal volume and surface area as well as percentage of uninstrumented canal wall sur- face and accumulated hard-tissue debris (AHTD) after canal preparation were compared statistically using the independent sample t-test and Mann–Whitney U-test, with the significance level set at 5%.
Results: Volume, surface area and percentage of static voxels in either mesial or distal root canal systems were not significantly different between groups before or after root canal preparation (P > 0.05). After preparation, AHTD was not observed in the distal canal of both groups. However, in the mesial root canal system, the conventional irrigation group was associated with a significantly higher median percentage of AHTD (11.48%; IQR: 5.9–22.6; range: 1.86–41.98) than the EndoVac group (3.40%; IQR: 1.5–7.3; range: 0.82–12.84) (P < 0.05).
Conclusions: Neither irrigation protocol succeeded in rendering the mesial canal system free of AHTD; however, apical negative pressure irrigation resulted in lower levels of AHTD than conventional irrigation.
Introduction
Root canal treatment outcomes are dependent upon effective canal cleaning and disinfection procedures, which eliminate or control the causative agents of apical periodontitis. In terms of cleaning, instruments and irrigating solutions/regimens have been evaluated mostly for their ability to remove soft-tissue remnants using histological analysis (Walton 1976, Zuolo et al. 1992, Siqueira et al. 1997, Taha et al. 2010, De-Deus et al. 2011). Recently, several authors have also focused on the accumulation of hard-tissue debris (AHTD) in recesses, isthmuses, irregularities and ramifications using micro-computed tomographic (micro-CT) imaging (Paqué et al. 2009, 2011, 2012a, Robinson et al. 2013, De-Deus et al. 2015). This technology has permitted researchers to monitor and quantify the accumulation and removal of radiopaque debris in various areas of the root canal system (Robinson et al. 2012, De-Deus et al. 2014, 2015).
Hard-tissue debris is formed during the cutting action of instruments on dentine and can be packed in some areas of the root canal system. In infected canals, AHTD may contain bacteria and serve as a nidus for root canal reinfection. In addition, debris packed in the canal system may compromise thorough disinfection and filling (Metzger et al. 2010). Studies have shown that the current instrumentation techniques are unable to provide AHTD-free canals (Paqué et al. 2011, 2012a,b, Robinson et al. 2013, De-Deus et al. 2015). Because AHTD is usually generated during instrumentation, improving irrigation is conceivably the best way to prevent the formation or remove accumulated debris.
Numerous irrigating solutions and delivery systems have been used in root canal preparation (Gu et al. 2009, Haapasalo et al. 2014). Conventional or standard irrigation uses various needle types adapted to a disposable plastic syringe associated with apical positive pressure. The use of flexible irrigation needles, which should be inserted in the canal as close as possible to the working length, associated with large volumes of irrigants and frequent exchanges is strategies to enhance the cleaning and disinfecting effects of conventional irrigation (Chow 1983, Siqueira et al. 2000, Sedgley et al. 2005). The EndoVac system (SybronEndo, Orange, CA, USA) comprises a different irrigation regimen that involves apical negative pressure and is composed of 3 basic components: a master delivery tip, a plastic macrocannula and a stainless steel microcannula. The former is used to deliver and evacuate the irrigant concomitantly at the pulp chamber level, whilst the 2 cannulas are used deep in the canal in sequence to improve irrigation at the apical canal level. Because these cannulas are used to aspirate the irrigant, a current flow in an apical direction is created (Gu et al. 2009). The EndoVac system can be regarded as safe when used with NaOCl (Desai & Himel 2009), which is confirmed by studies showing low extrusion of NaOCl for EndoVac irrigation when compared to other regimens (Mitchell et al. 2010, Iriboz et al. 2015). As for cleaning and disinfecting effects, studies comparing EndoVac with conventional irrigation have shown inconclusive results. Whilst some authors reported better cleaning of the apical canal (Nielsen & Craig Baumgartner 2007, Siu & Baumgartner 2010) and superior bacterial elimination (Hockett et al. 2008) when using the EndoVac, other studies reported no significant differences in cleaning (Howard et al. 2011) or disinfection (Brito et al. 2009, Miller & Baumgartner 2010, Pawar et al. 2012).
Despite the considerable amount of research con- ducted on root canal irrigation using different methodologies (Haapasalo et al. 2014), to date only one study attempted to evaluate the reduction of AHTD in mandibular molar root canals using the EndoVac system (Freire et al. 2015). Therefore, the purpose of this ex vivo study was to evaluate the efficacies of root canal and isthmus debridement of apical positive (conventional irrigation) and negative (Endo-Vac system) pressure irrigation protocols after preparation of the root canals in mandibular molars using micro-CT imaging analysis. The null hypothesis tested was that there was no difference in the efficacy of these irrigation protocols in the reduction of AHTD within the root canal system of mandibular molars.
Material and methods
Specimen selection and preparation
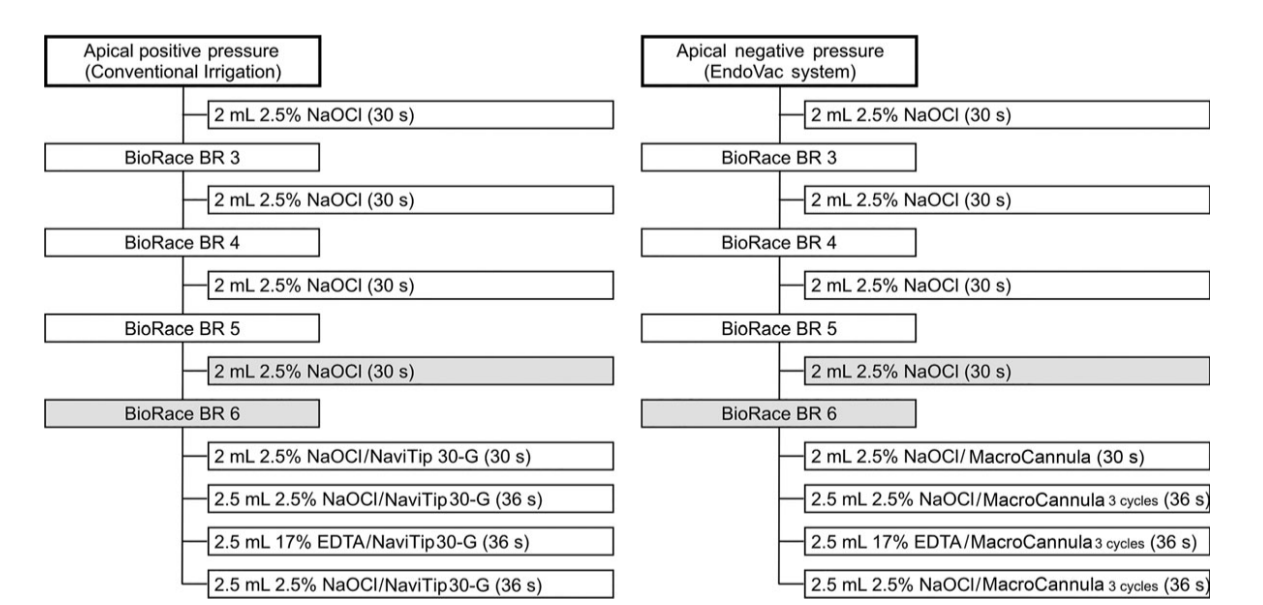
After ethics committee approval, one hundred and sixty decoronated two-rooted mandibular molars, extracted for reasons not related to this study, were imaged with a micro-CT scanner (SkyScan 1174v.2; Bruker-microCT, Kontich, Belgium) set at 50 kV, 800 µA, and an isotropic resolution of 19.89 lm. Scanning was performed through 180° rotation around the vertical axis with a rotation step of 1° using a 0.5-mm-thick aluminium filter. The acquired projection images were reconstructed into cross section slices (NRecon v.1.6.9; Bruker-microCT), and 3D models of the mesial and distal root canal systems were obtained and evaluated (CTVol v.2.2.1; Bruker-microCT). Twenty molar teeth with 2 independent canals connected by an isthmus from the middle to the apical third of the mesial root (type II Vertucci’s canal configuration) and a single canal in the distal root were selected. Morphological parameters of the canals (curvature, length, volume and surface area) were recorded (CTAn v.1.14.4; Bruker-microCT), and the specimens were pair-matched accordingly. Then, one tooth from each pair was randomly assigned to one of the 2 experimental groups, according to the chemomechanical procedure (Fig. 1).
After access cavity preparation, the working length (WL) was determined under magnification by introducing a size 10 K-type file into the canal until it reached the apical foramen. The WL was established
0.5 mm short of the foramen. Next, the apical foramen of each root was sealed with fast set epoxy resin to create a closed-ended system. Then, teeth were mounted vertically up to the cervical region in blocks made of a silicone impression material (President Jet, Coltène AG, Cuyahoga Falls, OH, USA) for the chemo- mechanical procedure steps. After initial irrigation with 2 mL of 2.5% NaOCl, all canals were enlarged using the BioRaCe rotary system (FKG Dentaire, La Chaux-de-Fonds, Switzerland) operated at 500 rpm, in a crown-down manner, up to BR5 (size 40, .04 taper) and BR6 (size 50, .04 taper) instruments in the mesial and distal root canals, respectively. The irrigation regimens were as follows (Fig. 1):
- Apical positive pressure group (n = 20 mesial canals and 10 distal canals): irrigation throughout the preparation procedures was performed using a 30-gauge NaviTip needle (Ultradent, South Jordan, UT, USA) adapted to a disposable plastic syringe placed up to 3 mm short of the WL in each canal using 2 mL of 2.5% NaOCl after each instrument. An additional 4.5 mL of 2.5% NaOCl followed by 2.5 mL of 17% EDTA and 2.5 mL of 2.5% NaOCl was used in the final irrigation.
- Apical negative pressure group (n = 20 mesial canals and 10 distal canals): each canal was irrigated with 2 mL of 2.5% NaOCl at each instrument change using the EndoVac master tip placed above the access opening. Following apical preparation, the plastic macrocannula (size 55, .02 taper) was inserted 3 mm short of the WL and irrigation was accomplished with 2 mL of NaOCl delivered coronally by the master tip over a 30-s period. Then, the pulp chamber was kept full of irrigant whilst the microcannula (size 32, .02 taper) was placed at WL for 6 s; next, the microcannula was positioned 2 mm from the WL for another 6 s. Three cycles of these up-and-down motions were accomplished, resulting in 36 s of NaOCl irrigation with the microcannula. This ‘microirrigation’ procedure was repeated using 17% EDTA as the irrigant (3 cycles) and then with NaOCl (3 cycles). At the end of the last cycle, the microcannula was left at the WL to remove excess irrigant.
The overall volume of NaOCl per group was 13 mL for each mesial canal and 15 mL for the distal canal. Total volume of EDTA was 2.5 mL per canal. The flow rate of irrigant was 2 mL per 30s and 2 mL per 36s during and after canal preparation, respectively (Fig. 1).
Micro-CT imaging analysis
3D models of the root canals after preparation were rendered and co-registered with their respective pre-operative data sets using the rigid registration module of the 3D Slicer 4.3.1 software (available from http:// www.slicer.org). Matched images of the canals were examined to calculate volume (mm3), surface area (mm2) and the amount of uninstrumented canal wall surface (static voxels) for both mesial and distal root canal systems (CTAn v.1.14.4; Bruker micro-CT). It was assumed that surface voxels remaining in the same place after root canal preparation (static voxels) represented uninstrumented aspects of the canal walls (Peters et al. 2001). Then, the percentage increase of volume and surface area parameters was calculated by subtracting the scores of the treated canals from those recorded for the untreated counterparts, for the full length of the canals and for the isthmus area of the mesial canal system. For the purpose of this study, the region of interest of the isthmus area also comprised the area of each mesial canal.
For the quantitative analysis of AHTD, the label masks of the registered data sets of each tooth were imported into the Fiji software (Fiji v.1.47n; Madison, WI, USA) and normalized. The sequence of images resulting from this operation was further used to identify the AHTD by means of morphologic operations. Quantification of AHTD was performed by the difference between nonprepared and prepared root canal space using post-processing procedures, expressed as the percentage of the total canal system volume after preparation. The presence of a material with density similar to dentine in regions previously occupied by air in the nonprepared root canal space was considered debris and quantified by intersection between images before and after canal instrumentation (Robinson et al. 2012). Colour-coded root canal models (green and red colours indicating pre- and postoperative canal surfaces, respectively) and debris (in black colour) enabled qualitative comparison of the matched root canals before and after preparation.
Statistical analysis
The Shapiro–Wilk test was used to assess the normality of the data. Before root canal preparation, volume and surface area values from either root canal or isthmus region had a normal distribution and were compared between groups using independent sample t-test. Obtained data after canal preparation (volume, surface area, static voxel and AHTD) were skewed and expressed as median and interquartile range (IQR). Statistical comparison between groups was performed using nonparametric Mann–Whitney U-test, and the significance level was set at 5%.
Results
The results of volume, surface area and static voxel parameters evaluated in both groups, before and after preparation, are detailed in Table 1. Pre- and postoperatively, the degree of homogeneity (baseline) of the groups with respect to length, volume and surface area of the root canals and the isthmus was confirmed (P > 0.05). Additionally, the percentage of static voxels after preparation of the root canals showed no significant difference between groups (P > 0.05).
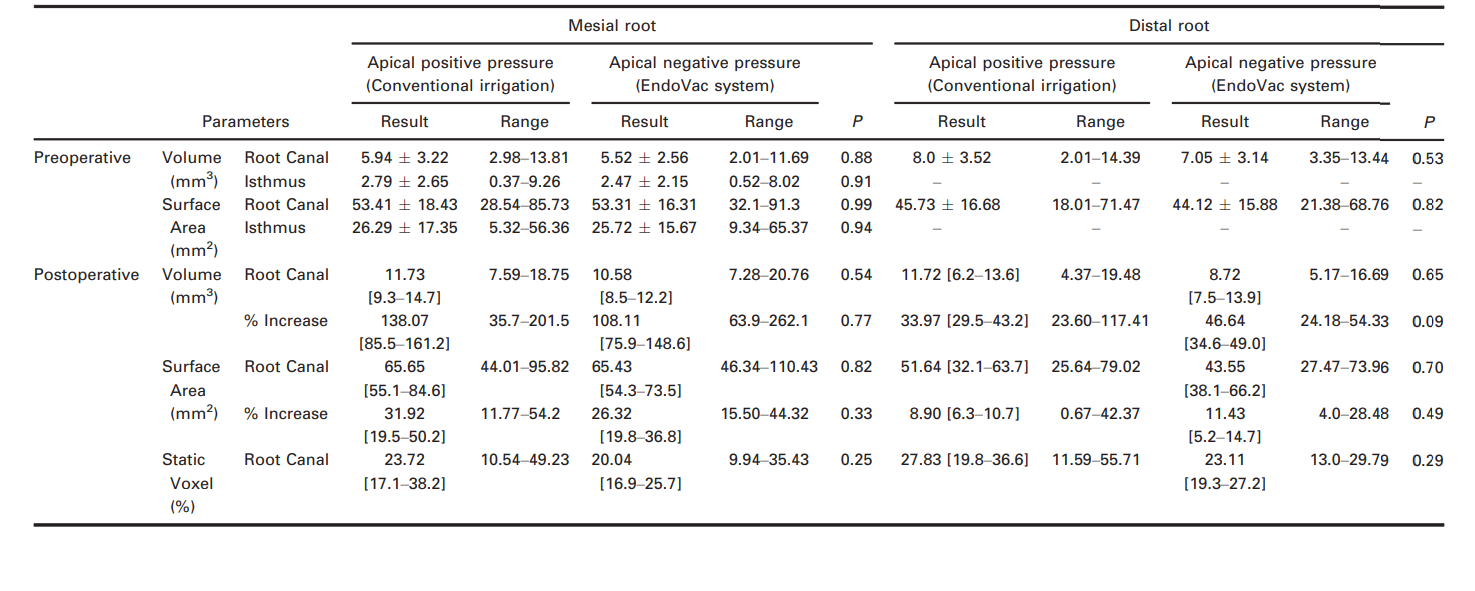
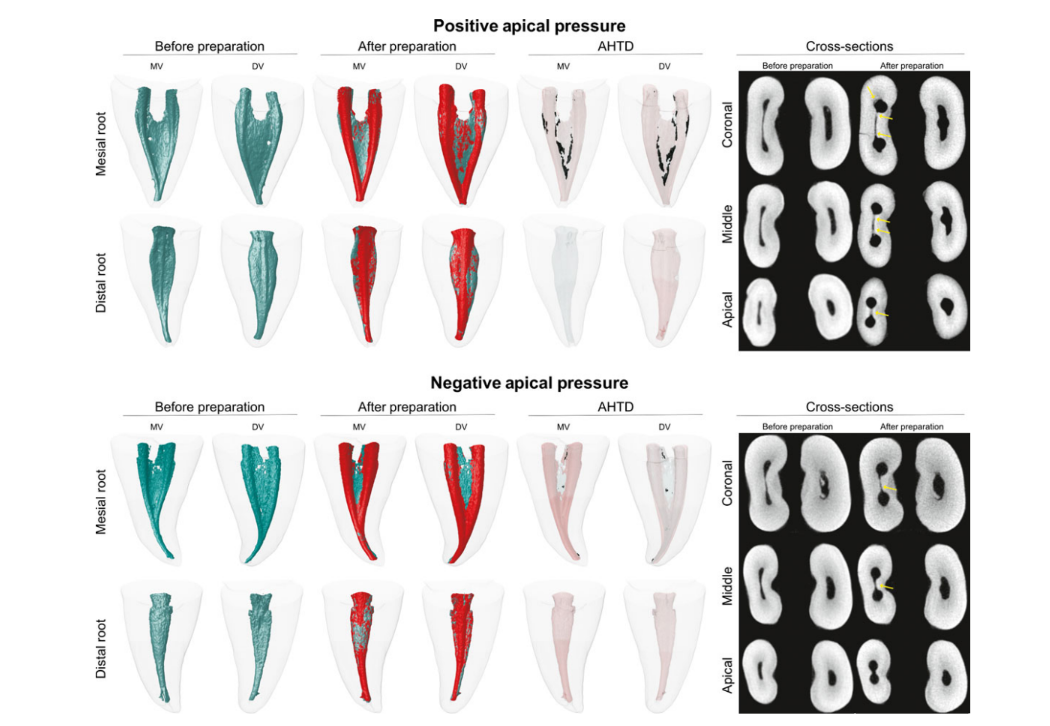
Figure 2 shows the distribution of the AHTD after root canal preparation using positive and negative pressure irrigation protocols in two representative mandibular molars. After preparation, AHTD was not observed in the distal canal of both groups. In the mesial root canal system, the conventional irrigation group had a significantly higher median percentage of AHTD (11.48%; IQR: 5.9–22.6; range: 1.86–41.98) than the EndoVac group (3.40%; IQR: 1.5–7.3; range: 0.82–12.84) (P < 0.05) (Fig. 2). Overall, AHTD was observed in the middle and apical root canal thirds after conventional irrigation, whilst negative pressure left AHTD mostly in the coronal third. Thus, the null hypothesis tested was rejected.
Discussion
Accumulated hard-tissue debris may potentially interfere with disinfection by preventing irrigant flow and neutralizing the antibacterial effects of the irrigating solution (Paqué et al. 2012a). In addition, it may interfere with canal filling by physically precluding the filling material from reaching some areas of the root canal system (Metzger et al. 2010, Freire et al. 2015). It has been hypothesized that the dentine particles cut from the canal walls by rotary instruments are actively packed into soft-tissue remnants of the hard-to-reach areas of the root canal and become more resistant to removal by conventional syringe-and-needle irrigation (Paqué et al. 2009, 2012a). To date, only a few studies have attempted to investigate different irrigation schemes for the ability to reduce AHTD in the root canal system of mandibular molars (Paqué et al. 2011, 2012a,b). Overall, the main conclusion of these studies was that sequential or supplementary irrigation procedures during or after root canal preparation resulted in less AHTD in isthmus-containing root canal systems. Using micro-CT technology, it was recently reported that the percentage volume of AHTD reduced from 4.10% to 2.12% (percentage reduction of 53.65%) after a final irrigation protocol using the EndoVac System in the mesial root canal system of mandibular molars (Freire et al. 2015), which is in accordance with the present results.
Findings from the present study revealed that both irrigation protocols succeeded in rendering distal root canals free of AHTD demonstrating the efficacy of the conventional irrigation regimen when used in root canals with single anatomy. However, despite its significantly better performance, EndoVac was not capable of completely removing AHTD from the mesial root canal system of mandibular molar teeth. In this anatomical canal configuration, the median percentage of AHTD was significantly lower in canals irrigated with EndoVac (3.4%) when compared with conventional irrigation (11.48%). These results may be explained by the mechanical flushing action created by the EndoVac system, which is more likely to remove debris from the hard-to-reach areas of the root canal compared with conventional syringe needle irrigation (Shin et al. 2010, Siu & Baumgartner 2010).
Root canal cleaning and disinfection occurs as a combination of the mechanical effects of preparation and the chemomechanical effects of irrigation. As the irrigating process is aided by the mechanical flushing action, the rate of irrigant flow plays an important role for removing the debris from within the root canal space, during and after root canal preparation. In the present study, the irrigant flow rate was set at
0.066 mL s—1 (Fig. 1). Even though some authors have recommend the use of higher flow rates in positive irrigation protocols (Boutsioukis et al. 2007, Khan et al. 2013), the flow rate of 0.066 mL s—1 was chosen in the present study because the impossibility of applying a higher flow rate with apical negative pressure systems (Brunson et al. 2010). Consequently, it may be argued that such a low rate for syringe irrigation may have biased the results in favour of the apical negative pressure system. However, in a recent in vitro study, it was showed that the use of positive pressure irrigation with a flow rate of 4 mL min—1 (or 0.066 mL s—1 as in the present study) was able to achieve maximum effectiveness (Park et al. 2013).
It is important to point out that the experimental groups in this study differed not only in the mode of irrigant delivery (positive vs. negative pressure), but also in the delivery protocol, which was not possible to standardize. Actually, this lack of standardization is a very common problem with studies using EndoVac system protocol, because the irrigant is not delivered within the root canal system, but in the pulp chamber. In the syringe irrigation group, the open-ended needle tip was positioned 3 mm from the WL following the EndoVac plastic macrocannula insertion depth. This level was also chosen because previous studies have reported that it may improve irrigant replacement and wall shear stress (Shen et al. 2010), while reducing the risk of wedging and irrigant extrusion (Boutsioukis et al. 2014). On the other hand, in the second step of the irrigation procedure in the negative pressure group, EndoVac microcannula was placed at the WL, following manufacturer’s recommendations. Thus, despite the reported results reflect true differences between the tested protocols, it remains unclear in which magnitude the difference in the irrigant delivery affected the results (Adorno et al. 2015), and further studies are required.
The mechanical action of instruments on canal walls includes the removal of the inner layer of infected dentine to remove or disrupt bacterial biofilms (Paqué & Peters 2011), which may improve the outcome of the root canal treatment. In the present study, the percentage of uninstrumented root canal walls was expressed as a percentage of the number of static voxel surface to the total number of surface voxels (Peters et al. 2001). Previous studies have reported that the mean percentage of untreated areas after preparation with different rotary systems ranged from 59% to 79% in long oval-shaped distal canals (Paqué et al. 2010) and from 39% to 42% in independent mesial canals of mandibular molars (Yang et al. 2011). These high percentage values were associated with the kinematics of rotary instruments (pecking motion) and the presence of recesses in long oval-shaped canals, which were not included in the rounded preparation created by the rotation of instruments. In the present study, median percentage values of unprepared areas were not different between the groups in the distal canal (27.83% and 23.11%) and in the mesial root canal system when the isthmus was not included in the analysis (23.72% and 20.04%, respectively). These lower percentage values compared to those previously reported might be explained as the consequence of differences in the preparation protocol, instrument sizes and the root canal configuration used herein.
The main role of laboratory-based studies is to develop well-controlled conditions that are able to reliably compare certain factors (Versiani et al. 2013). One of the most important confounding factors in ex vivo studies is the anatomy of the root canal system under investigation. Consequently, the results might reflect the effects of canal anatomy rather than the variable of interest (Peters et al. 2001). In the present study, pair-wise distribution of the specimens based on the configuration and morphology (length, volume and surface area) of the mesial and distal root canal systems probably eliminated or, at least, substantially reduced potentially significant anatomical biases that could interfere with the results. Therefore, no differences regarding volume, surface area and percentage of static voxels were observed before or after root canal preparation between the experimental groups in which the same mechanical preparation protocol was used (Table 1). Thus, based on micro-CT data, it is possible to improve sample selection using established morphological parameters to provide a consistent baseline, which enhances the internal validity of ex vivo experiments (Versiani et al. 2013, Marceliano-Alves et al. 2015).
Conclusions
Accumulated hard-tissue debris was not observed in the distal canals of mandibular molars. Neither irrigation approach succeeded in rendering the mesial canal system free of AHTD. Apical negative pressure irrigation resulted in significantly lower levels of AHTD compared with conventional irrigation only in the mesial root canal system.
Authors: M. A. Versiani, F. R. F. Alves, C. V. Andrade-Junior, M. F. Marceliano-Alves, J. C. Provenzano, I. N. Rôças, M. D. Sousa-Neto & J. F. Siqueira Jr
References:
- Adorno CG, Fretes VR, Ortiz CP et al. (2015) Comparison of two negative pressure systems and syringe irrigation for root canal irrigation: an ex vivo study. International Endodontic Journal doi: 10.1111/iej.12431 [Epub ahead of print].
- Boutsioukis C, Lambrianidis T, Kastrinakis E, Bekiaroglou P (2007) Measurement of pressure and flow rates during irrigation of a root canal ex vivo with three endodontic needles. International Endodontic Journal 40, 504–13.
- Boutsioukis C, Psimma Z, Kastrinakis E (2014) The effect of flow rate and agitation technique on irrigant extrusion ex vivo. Journal of Endodontics 47, 487–96.
- Brito PR, Souza LC, Machado de Oliveira JC et al. (2009) Comparison of the effectiveness of three irrigation techniques in reducing intracanal Enterococcus faecalis populations: an in vitro study. Journal of Endodontics 35, 1422–7. Brunson M, Heilborn C, Johnson DJ, Cohenca N (2010) Effect of apical preparation size and preparation taper on irrigant volume delivered by using negative pressure irrigation system. Journal of Endodontics 36, 721–4.
- Chow TW (1983) Mechanical effectiveness of root canal irrigation. Journal of Endodontics 9, 475–9.
- De-Deus G, Souza EM, Barino B et al. (2011) The self-adjusting file optimizes debridement quality in oval-shaped root canals. Journal of Endodontics 37, 701–5.
- De-Deus G, Roter J, Reis C et al. (2014) Assessing accumulated hard-tissue debris using micro-computed tomography and free software for image processing and analysis. Journal of Endodontics 40, 271–6.
- De-Deus G, Marins J, Silva EJ et al. (2015) Accumulated hard-tissue debris produced during reciprocating and rotary nickel-titanium canal preparation. Journal of Endodontics 41, 676–81.
- Desai P, Himel V (2009) Comparative safety of various intracanal irrigation systems. Journal of Endodontics 35, 545–9. Freire LG, Iglecias EF, Cunha RS, dos Santos M, Gavini G (2015) Micro-computed tomographic evaluation of hard tissue debris removal after different irrigation methods and its influence on the filling of curved canals. Journal of Endodontics 41, 1660–6.
- Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR (2009) Review of contemporary irrigant agitation techniques and devices. Journal of Endodontics 35, 791–804.
- Haapasalo M, Shen Y, Wang Z, Gao Y (2014) Irrigation in endodontics. British Dental Journal 216, 299–303.
- Hockett JL, Dommisch JK, Johnson JD, Cohenca N (2008) Antimicrobial efficacy of two irrigation techniques in tapered and nontapered canal preparations: an in vitro study. Journal of Endodontics 34, 1374–7.
- Howard RK, Kirkpatrick TC, Rutledge RE, Yaccino JM (2011) Comparison of debris removal with three different irrigation techniques. Journal of Endodontics 37, 1301–5.
- Iriboz E, Bayraktar K, Turkaydin D, Tarcin B (2015) Comparison of apical extrusion of sodium hypochlorite using 4 different root canal irrigation techniques. Journal of Endodontics 41, 380–4.
- Khan S, Niu LN, Eid AA et al. (2013) Periapical pressures developed by nonbinding irrigation needles at various irrigation delivery rates. Journal of Endodontics 39, 529–33.
- Marceliano-Alves MF, Sousa-Neto MD, Fidel SR et al. (2015) Shaping ability of single-file reciprocating and heat-treated multifile rotary systems: a micro-CT study. International Endodontic Journal, 48, 1129–36.
- Metzger Z, Zary R, Cohen R, Teperovich E, Paqué F (2010) The quality of root canal preparation and root canal obturation in canals treated with rotary versus self-adjusting files: a three-dimensional micro-computed tomographic study. Journal of Endodontics 36, 1569–73.
- Miller TA, Baumgartner JC (2010) Comparison of the antimicrobial efficacy of irrigation using the EndoVac to endodontic needle delivery. Journal of Endodontics 36, 509–11.
- Mitchell RP, Yang SE, Baumgartner JC (2010) Comparison of apical extrusion of NaOCl using the EndoVac or needle irrigation of root canals. Journal of Endodontics 36, 338– 41.
- Nielsen B, Craig Baumgartner J (2007) Comparison of the EndoVac system to needle irrigation of root canals. Journal of Endodontics 33, 611–15.
- Paqué F, Peters OA (2011) Micro-computed tomography evaluation of the preparation of long oval root canals in mandibular molars with the self-adjusting file. Journal of Endodontics 37, 517–21.
- Paqué F, Laib A, Gautschi H, Zehnder M (2009) Hard-tissue debris accumulation analysis by high-resolution computed tomography scans. Journal of Endodontics 35, 1044–7.
- Paqué F, Balmer M, Attin T, Peters OA (2010) Preparation of oval-shaped root canals in mandibular molars using nickel-titanium rotary instruments: a micro-computed tomography study. Journal of Endodontics 36, 703–7.
- Paqué F, Boessler C, Zehnder M (2011) Accumulated hard tissue debris levels in mesial roots of mandibular molars after sequential irrigation steps. International Endodontic Journal 44, 148–53.
- Paqué F, Al-Jadaa A, Kfir A (2012a) Hard-tissue debris accumulation created by conventional rotary versus self-adjusting file instrumentation in mesial root canal systems of mandibular molars. International Endodontic Journal 45, 413–18.
- Paqué F, Rechenberg DK, Zehnder M (2012b) Reduction of hard-tissue debris accumulation during rotary root canal instrumentation by etidronic acid in a sodium hypochlorite irrigant. Journal of Endodontics 38, 692–5.
- Park E, Shen Y, Khakpour M, Haapasalo M (2013) Apical pressure and extent of irrigant flow beyond the needle tip during positive-pressure irrigation in an in vitro root canal model. Journal of Endodontics 39, 511–15.
- Pawar R, Alqaied A, Safavi K, Boyko J, Kaufman B (2012) Influence of an apical negative pressure irrigation system on bacterial elimination during endodontic therapy: a prospective randomized clinical study. Journal of Endodontics 38, 1177–81.
- Peters OA, Laib A, Gohring TN, Barbakow F (2001) Changes in root canal geometry after preparation assessed by high-resolution computed tomography. Journal of Endodontics 27, 1–6.
- Robinson JP, Lumley PJ, Claridge E et al. (2012) An analytical Micro-CT methodology for quantifying inorganic dentine debris following internal tooth preparation. Journal of Dentistry 40, 999–1005.
- Robinson JP, Lumley PJ, Cooper PR, Grover LM, Walmsley AD (2013) Reciprocating root canal technique induces greater debris accumulation than a continuous rotary technique as assessed by 3-dimensional micro-computed tomography. Journal of Endodontics 39, 1067–70.
- Sedgley CM, Nagel AC, Hall D, Applegate B (2005) Influence of irrigant needle depth in removing bioluminescent bacteria inoculated into instrumented root canals using real-time imaging in vitro. International Endodontic Journal 38, 97–104.
- Shen Y, Gao Y, Qian W et al. (2010) Three-dimensional numeric simulation of root canal irrigant flow with different irrigation needles. Journal of Endodontics 36, 884–9.
- Shin SJ, Kim HK, Jung IY, Lee CY, Lee SJ, Kim E (2010) Comparison of the cleaning efficacy of a new apical negative pressure irrigating system with conventional irrigation needles in the root canals. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics 109, 479–84.
- Siqueira JF Jr, Araújo MC, Garcia PF, Fraga RC, Dantas CJ (1997) Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. Journal of Endodontics 23, 499–502.
- Siqueira JF Jr, Rocas IN, Favieri A, Lima KC (2000) Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. Journal of Endodontics 26, 331–4.
- Siu C, Baumgartner JC (2010) Comparison of the debridement efficacy of the EndoVac irrigation system and conventional needle root canal irrigation in vivo. Journal of Endodontics 36, 1782–5.
- Taha NA, Ozawa T, Messer HH (2010) Comparison of three techniques for preparing oval-shaped root canals. Journal of Endodontics 36, 532–5.
- Versiani MA, Pécora JD, Sousa-Neto MD (2013) Microcomputed tomography analysis of the root canal morphology of single-rooted mandibular canines. International Endodontic Journal 46, 800–7.
- Walton RE (1976) Histologic evaluation of different methods of enlarging the pulp canal space. Journal of Endodontics 2, 304–11.
- Yang G, Yuan G, Yun X, Zhou X, Liu B, Wu H (2011) Effects of two nickel-titanium instrument systems, Mtwo versus ProTaper universal, on root canal geometry assessed by micro-computed tomography. Journal of Endodontics 37, 1412–16.
- Zuolo ML, Walton RE, Imura N (1992) Histologic evaluation of three endodontic instrument/preparation techniques. Endodontics G Dental Traumatology 8, 125–9.
/social-network-service/media/default/6758/89a8282e.png)