Low Satellite DNA Variability in Natural Populations of Drosophila antonietae Involved in Different Evolutionary Events
Drosophila antonietae is a cactophilic species that is found in the mesophilic forest of the Paraná–Paraguay river basin and in the dunes of the South Atlantic coast of Brazil. Although the genetic structure of the Paraná–Paraguay river basin populations has already been established, the relationship between these populations and those on the Atlantic coast is controversial. In this study, we compared 33 repetitive units of pBuM-2 satellite DNA isolated from individuals from 8 populations of D. antonietae in these geographic regions, including some populations found within a contact zone with the closely related D. serido. The pBuM-2 sequences showed low interpopulational variability. This result was interpreted as a consequence of both gene flow among the populations and unequal crossing over promoting homogenization of the tandem arrays. The results presented here, together with those of previous studies, highlight the use of pBuM-2 for solving taxonomic conflicts within the D. buzzatii species cluster.
Introduction
Drosophila antonietae is a member of the D. buzzatii species cluster, a monophyletic group composed of 7 sibling species naturally endemic to the neotropical region occurring in the open and dry forest found in eastern South America (Manfrin and Sene 2006). These species use necrotic cactus tissue as breeding sites (Pereira et al. 1983); because of this, their geographic range is restricted to the distribution range of the host cacti. Thus, D. antonietae is an attractive model in which to study evolutionary processes (Manfrin and Sene 2006).
The geographic distribution of D. antonietae can be characterized in 2 ways. In the valleys enclosed by the Paraná–Paraguay river basin, populations are found in mesophilic forests associated with the cactus Cereus hildemannianus (Tidon-Sklorz and Sene 2001; Mateus and Sene 2003). In its south-east distribution, D. antonietae occurs in the dune regions of the Atlantic coast, using primarily C. hildemannianus but also cacti from the genus Opuntia (Ruiz et al. 2000; Manfrin and Sene 2006) (Figure 1).
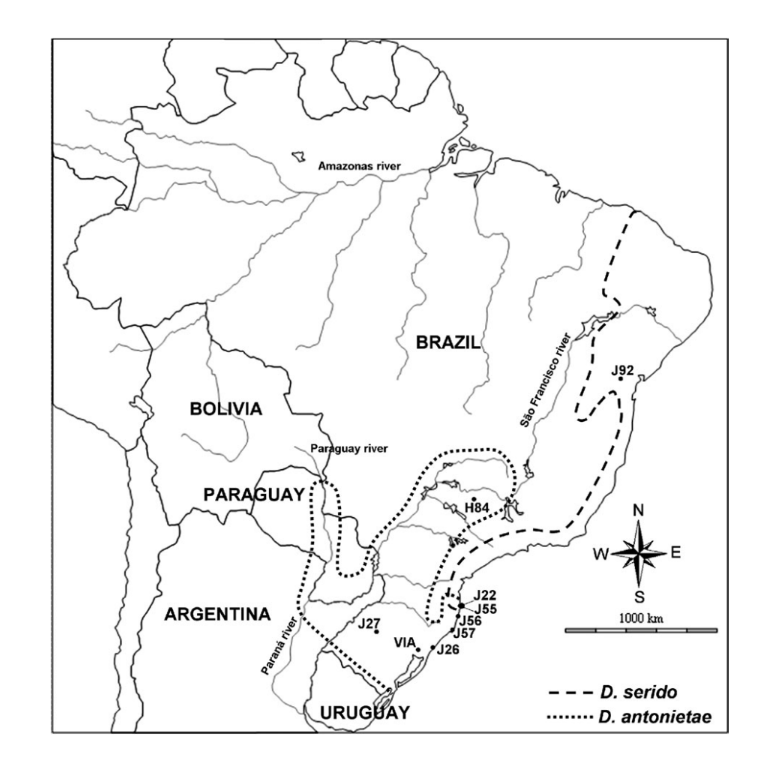
Previous studies have suggested that populations of D. antonietae from the Paraná–Paraguay river basin are structured according to the isolation-by-distance model due to the positive correlation of morphological and genetic distances with geographical and ecological distances (the latter defined as a distance between 2 populations following the course of the river) (de Brito et al. 2002; Mateus and Sene 2007). Based on the number of migrants per generation, Mateus and Sene (2007) suggested a moderate effect of both gene flow and genetic drift in these populations of D. antonietae with gene flow overlapping genetic drift promoting a genetic gradient.
A phylogeographic analysis based on mitochondrial DNA suggested that the southeast distribution of D. antonietae, on the Atlantic coast of Brazil, was colonized by expansion events of D. antonietae populations from the central depression of the Rio Grande do Sul state (de Brito et al. 2002; Manfrin and Sene 2006). However, this analysis could not determine whether this expansion was followed by maintenance of gene flow, with acquisition of population structure according to the isolation-by-distance model, or followed by fragmentation, implicating an absence of gene flow between the populations of the Atlantic coast and those from the Paraná–Paraguay river basin (de Brito et al. 2002). The chromosome inversion data might suggest some degree of fragmentation between the populations from these 2 different environments, as those populations from the valleys of the Paraná–Paraguay river basin are polymorphic for inversions 2y8, 2z8 and 5e, whereas the populations from the Atlantic coast are polymorphic for 2z8 (Ruiz et al. 2000). The Atlantic coast populations of D. antonietae have another important characteristic: they are in contact with the closely related species D. serido (D. buzzatii cluster) (Ruiz et al. 2000; Manfrin and Sene 2006). This area of overlap is the result of D. serido expanding from the north to the south Atlantic coastline and sharing a geographic locale with
D. antonietae populations (de Brito et al. 2002; Manfrin and Sene 2006) (Figure 1). Morphological traits and mitochondrial DNA analyses suggest that hybridization between
D. antonietae and D. serido has occurred within the contact zone (C.K.B. Santos and colleagues, unpublished data).
Satellite DNAs are highly repetitive tandemly arranged DNA sequences that represent a substantial component of the heterochromatin of eukaryotic organisms (Charlesworth et al. 1tt4; Palomeque and Lorite 2008). Satellites are typically located near centromeric regions and, less frequently, in telomeres (Ugarkovic and Plohl 2002; Kuhnet al. 2008). These sequences evolve in a concerted manner, whereas the main molecular mechanisms involved in their evolution are slippage replication, unequal crossing over, gene conversion, and rolling circle replication (Dover 1982; Charlesworth et al. 1994).
Some of the satellite DNA families evolve quickly, allowing their use as molecular markers in interspecific comparative studies (Watabe et al. 1997; Picariello et al. 2002; Kuhn and Sene 2005). However, the use of these sequences as molecular markers in population studies has been relatively underexplored, despite the fact that concerted evolution was reported in populations of Cyprinodon variegates (Cyprinodontidae) (Elder and Turner 1994) and Acrossocheilus paradoxus (Cyprinidae) (Wu et al. 1999).
Three satellite DNA families shared among the species of D. buzzatii cluster have been formally described: pBuM (Kuhn et al. 1999, 2008; Kuhn and Sene 2005), DBC-150 (Kuhn et al. 2007), and SSS139 (Franco et al. 2008). The pBuM family is composed of 2 subfamilies, pBuM-1 and pBuM-2 (Kuhn and Sene 2005; Kuhn et al. 2008). The pBuM-2 subfamily is composed of AT-rich monomers of 370 bp, which presents qualitative (fixed nucleotide substitution) and quantitative (copy number) differences among the species of the D. buzzatii cluster (Kuhn and Sene 2005; Kuhn et al. 2007, 2008).
In this work, we demonstrate the intraspecific variability of the pBuM-2 satellite DNA in D. antonietae, using populations from 2 different environments (the Paraná– Paraguay basin and the Atlantic coast) that present distinct evolutionary histories, associated with maintenance of gene flow in the former and hybridization events with the closely related species D. serido in the latter. We chose to use the pBuM satellite DNA as a molecular marker because it exhibits 14 diagnostic nucleotide substitutions between D. antonietae and D. serido (Kuhn and Sene 2005). Thus, pBuM allows the taxonomic identification of specimens isolated from the contact zone. Moreover, as the evolutionary dynamics of satellite DNA sequences at the intraspecific level are not completely understood, further empirical data might contribute to a better understanding of the process underlying satellite DNA evolution (Ugarkovicand Plohl 2002; Palomeque and Lorite 2008).
Material and Methods
Samples
We studied 8 Brazilian populations of D. antonietae (Figure 1). Three of these populations are located in the valleys of Paraná–Paraguay river basin: J2/ (Santiago, Rio Grande do Sul state), Via (Viamão, Rio Grande do Sul state), and H84 (Serrana, São Paulo state, accession number AY656609– AY656615; Kuhn and Sene 2005); and 5 are located in the dunes of Atlantic coast, within the contact zone with
D. serido: J56 (Garopaba, Santa Catarina state), J55 (Armaxcão beach, Floriano´polis, Santa Catarina state), J22 (Joaquina beach, Florianópolis, Santa Catarina state), J5/ (Laguna, Santa Catarina state), and J26 (Osório, Rio Grande do Sul state) (Figure 1). The sequences of D. antonietae were analyzed together with G pBuM-2 sequences from D. serido (population Jf2—Milagres, Bahia state, accession number AY656616–AY656621; Kuhn and Sene 2005), in order to identify some introgression events in populations from Atlantic coast.
Isolation of pBuM-2 Satellite DNA and Sequence Analysis
Genomic DNA from one male individual (wild-caught flies) per population was extracted using the Wizard Genomic DNA Purification Kit (Promega). The genomic DNA was submitted to polymerase chain reaction to amplify the copies of pBuM-2 family [primers A2F (CGGAGTA-TTTTTCATTCGAC) and A2R (GGTATGCCATAAAG-AAGTCG)] according to Kuhn et al. (2008). The resultant bands of approximately 400 bp were eluted from the gel by overnight incubation in an elution solution (500 mM NaAc; 1 mM ethylenediaminetetraacetic acid). The recovered fragments were cloned using the pMOSBlue blunt ended cloning kit (RPN 5110; Amersham Pharmacia Biotech). Plasmid DNA was prepared following the methodology described in Sambrook et al. (1989), and the DNA template reaction for sequencing was prepared according to the BigDye Terminator Cycle Sequencing Ready Reaction Kit manual (PerkinElmer). Automatic DNA sequencing was performed on an ABI Prism 377 sequencer (PerkinElmer).
Sequence alignments were carried out in CLUSTALW 1.8 (Thompson et al. 1994) and edited in BioEdit (Hall 1999). We used the algorithm and the statistical model proposed by Betrán et al. (1997) to detect gene conversion tracts. The parameter Rm, which estimates the minimum number of recombination events in a DNA sample (Hudson and Kaplan 1985), was used to detect recombination events. Although this parameter does not specifically measure unequal crossing over, it is reasonable to assume that the Rm would have values higher than zero if unequal crossing had occurred. In this sense, we use Rm as indirect evidence of unequal crossing over. The genetic distances were calculated according to p-distance, which is the proportion of nucleotide differences between 2 sequences (i.e., p 5 Np/N, where Np is the nucleotides’ differences and N is the total number of nucleotides). More complex distance models were also applied to the data, with similar results as those presented by p-distance (data not shown). The distance matrix was used to create a phenogram using the neighbor joining method (Saitou and Nei 1987). Genetic distance calculations and phenograms were made using the MEGA 3.0 program (Kumar et al. 2004).
We also investigated patterns of intraspecific variation in D. antonietae by means of analyses of molecular variance (AMOVAs; Excoffier et al. 1992). For AMOVA, populations of D. antonietae were clustered in 2 groups: Atlantic coast and Paraná–Paraguay river basin. The AMOVA was performed using Arlequin 3.0 (Excoffier et al. 2005).
Results and Discussion
Twenty-six new pBuM-2 satellite DNA monomers were obtained: 4 from locality J56 (accession number FJ935973– FJ93597G); 4 from locality Via (accession number FJt35t77–FJt35t80); 4 from locality J26 (accession number FJ935981–FJ935984); 3 from locality J5/ (accession number FJ935985–FJ935987); 5 from locality J2/ (accession number FJ935988–FJ935992); 2 from locality J55 (accession number FJ935993–FJ935994); and 4 from locality J22 (accession number FJ935995–FJ935998). The high nucleotide similarity among the sequences obtained in this work and those previously described (Kuhn and Sene 2005) confirms that they belong to the pBuM-2 satellite DNA family.
All sequences obtained present the primary structure typical of D. antonietae and were grouped in the same branch in the phenogram, together with the previously described pBuM-2 sequences of D. antonietae (Figure 2), even those from the populations within the contact zone with D. serido, where it was suggested hybridization between D. antonietae and D. serido with introgression of mitochondrial DNA (C.K.B Santos, F.M. Sene, and M.H. Manfrin, unpublished data). This situation could be related to the observations that mitochondrial genes tend to introgress more easily than the nuclear sequences (Dorado et al. 1992; Arnold 1993). The most cited explanations for this observation are related to cytonuclear disequilibria processes (Arnold 1993) and sterility of hybrid males (Aubert and Solignac 1990).
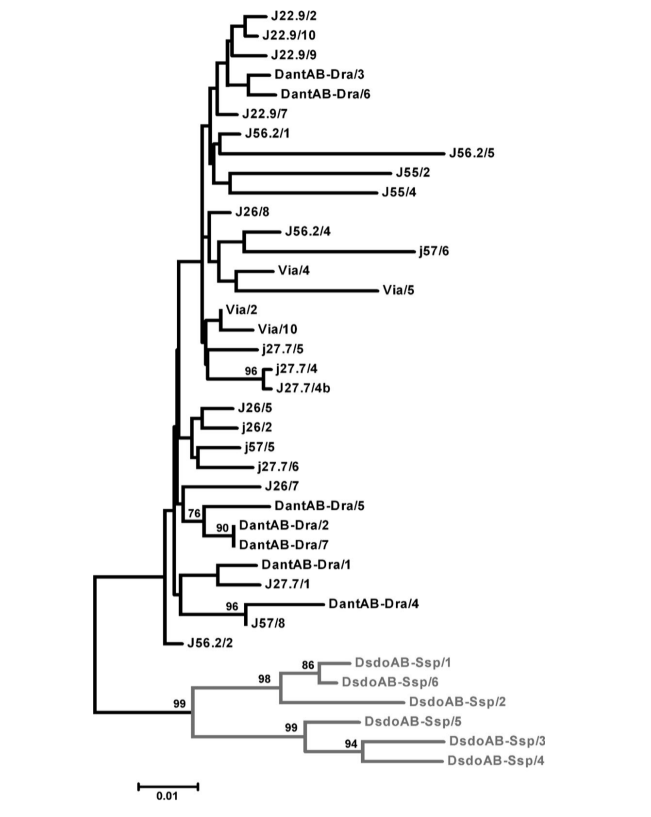
There is high level of sequence similarity among the pBuM-2 sequences of D. antonietae (95.7% on average), and no mutations were found to be diagnostic for a particular population. The interpopulational variability was very similar to the intrapopulational variability (Table 1), and sequences from the same population did not cluster together in a specific branch in the dendrogram (Figure 2). AMOVA performed with the populations subdivided by geographic groups (Paraná–Paraguay river basin and Atlantic coast) showed that 99.30% of the total genetic diversity could be ascribed to intragroup variability, whereas just 0.70% was ascribed to intergroup variability (Table 2). The Фst calculated was low and did not have statistical support (Table 2).
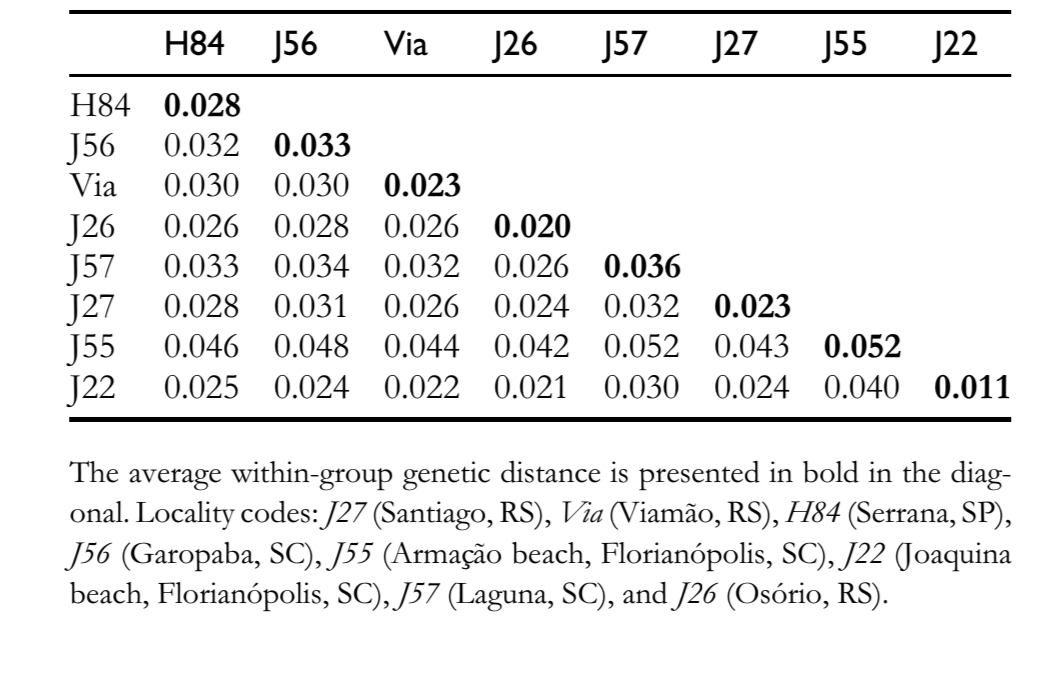

The populations of D. antonietae from the Paraná–Paraguay river basin are structured according to an isolation-by-distance model (Monteiro and Sene 1995; Mateus and Sene 2007). The observed chromosome inversions suggest fragmentation between the populations from Atlantic coast and those from the Paraná–Paraguay river basin (Ruiz et al. 2000), whereas mitochondrial DNA data could not conclude whether fragmentation or isolation-by-distance was the dominant structure defining the relationship of these 2 population groups (de Brito et al. 2002). The high nucleotide similarity of pBuM-2 in D. antonietae (Figure 2 and Tables 1 and 2) is congruent with maintenance of gene flow among the populations of this species, even among those allocated in the Parana´–Paraguay river basin and Atlantic coast. Our results suggest that isolation-by-distance should explain the mitochondrial DNA variability in D. antonietae; this model predicts gene flow between the populations, as inferred based on the satellite DNA data present here. In this context, the lowest chromosome inversion polymorphisms in the populations from Atlantic coast of D. antonietae, detected by Ruiz et al. (2000), might be explained as a consequence of historical and demographic events such as the population expansion from populations of the valleys of the Paraná–Paraguay river basin, as suggested by mitochondrial DNA (de Brito et al. 2002). Alternatively, this population differentiation might be associated to local adaptation to distinct environment in the presence of gene flow as chromosome inversions may be correlated with adaptive characteristics in cactophilic Drosophila (Hasson et al. 1992; Fernández Iriarte et al. 2003). An alternative explanation for the high nucleotide conservation of pBuM-2 in D. antonietae is based on the evolutionary dynamics of satellite DNA sequences. Molecular mechanisms related to the homogenization of tandemly arranged sequences, such as unequal crossing over and gene conversion through a process called molecular drive (Dover 1982; Strachan et al. 1985), could be acting on the pBuM satellite DNA in this species. Our analyses identified no gene conversion tracts in the pBuM-2 sequences isolated from D. antonietae, suggesting that this molecular mechanism does not influence pBuM-2 sequence diversity in this species. However, a minimum of 4 recombination events was detected in the sample (Rm = 4) between the intervals of 130–136, 136–155, 174–376, and 376–378. These recombination events could be an indirect evidence of unequal crossing over contributing to the maintenance of the primary structure of the pBuM-2 sequences in the different populations of D. antonietae. Consistent with this hypothesis, the D. antonietae genome contains a large number of pBuM-2 monomers (Kuhn et al. 2007; Kuhn et al. 2008), a fact that increases the probability of unequal crossing over (Smith1976), and consequently homogenization and concerted evolution.
These 2 alternative hypotheses explaining the high nucleotide similarity found in pBuM-2 monomers of D. antonietae are not mutually exclusive. In this context, we propose that a balance between gene flow among populations and homogenization events of the satellite DNA tandem arrays, promoted by unequal crossing over, contributes to the conservation of the pBuM-2 primary structure in D. antonietae species.
The absence of molecular divergence in satellite DNA sequences between populations of the same species, as found in the present paper, has also been observed in other insect species (Bachmann et al. 1994, 1998; Lorite et al. 2002; Feliciello et al. 2005). For example, Bachmann et al. (1994) found high levels of genetic similarity among monomers of the pDoP102 satellite in populations of Dolichopoda schiavazzii (Orthoptera), which allozymatic data indicate have been isolated from one another for at least 235 000 years. According to these authors, the homogeneity in pDoP102 even in isolated populations could be due to the biology of satellite DNA because unequal crossing over and gene conversion may induce the maintenance of primary structure of satellite DNA sequences (Bachmann et al. 1994). In other study, the presence of gene conversion tracts in the monomers of a satellite DNA family was used as evidence of homogenization events in this satellite DNA, explaining the high intraspecific satellite DNA conservation found in the phytophagous beetle Xanthogaleruca luteola (Lorite et al. 2002).
In the D. buzzatii cluster species, high intraspecific conservation of the pBuM-2 sequence was also identified in D. seriema (Kuhn and Sene 2004) and D. gouveai (Franco, Kuhn, et al. 2006). Moreover, the populations of D. buzzatii are homogenous with regards to pBuM-1 satellite DNA, another member of the pBuM family (Kuhn et al. 2003). In D. seriema and D. buzzatii, the lack of population differentiation in satellite DNA sequences was suggested to arise due to past and current gene flow events among populations, which are compatible with the results obtained from other genetic markers analyzed in the same populations (Kuhn et al. 2003; Kuhn and Sene 2004). For
D. gouveai, that is structured according to an ‘‘island’’ model (de Brito et al. 2002; Moraes and Sene 2007), the main hypothesis to explain the primary structure conservation in the pBuM-2 satellite DNA was that independent homogenization mechanisms favoring repetitive units shared among the populations occurred after the isolation event of the populations of D. gouveai (Franco, Kuhn, et al. 2006). The D. buzzatii cluster is composed of sibling species with taxonomy based on the morphometric characteristics of the aedeagus, which is the intromittent organ of male genitalia of insects (Vilela 1983; Tidon-Sklorz and Sene 2001; Franco, Prado, et al. 2006). Thus, the identification of female individuals of the D. buzzatii cluster, provided from the natural environment, is done by the identification of their male offspring making necessary the establishment of isofemale lines, which may be a hardy task for cactophilic Drosophila species. Moreover, the correctly species discrimination based only in quantitative characters, such as aedeagus morphology, sometimes is difficult due to the presence of individuals morphologically ambiguous, especially when the individuals are collected within contact zones between species of the D. buzzatii cluster. In this sense, the strong intraspecific conservation of pBuM satellite DNA sequences obtained in several studies (Kuhn et al. 2003; Kuhn and Sene 2004; Franco, Kuhn, et al. 2006; present paper), even in species genetically structured, highlight the utility of pBuM-2 in solving taxonomic conflicts within the D. buzzatii cluster species, an recognized model for evolutionary biology studies (Manfrin and Sene 2006).
Authors: Fernando Faria Franco, Fabio Melo Sene, Maura Helena Manfrin
References:
- Arnold J. 1993. Cytonuclear disequilibria in hybrid zones. Annu Rev Ecol Syst. 24:521–554.
- Aubert J, Solignac M. 1990. Experimental evidence for mitochondrial DNA introgression between Drosophila species. Evolution. 44:1272–1282.
- Bachmann L, Tomiuk J, Adis J, Vohland K. 1998. Genetic differentiation of the millipede Pycnotropis epiclysmus inhabiting seasonally inundated and non-flooded Amazonian forests. J Zool Syst Evol Res. 36:65–70.
- Bachmann L, Venanzetti F, Sbordoni V. 1994. Characterization of a species-specific satellite DNA family of Dolichopoda schiavazzi (Orthoptera, Rhaphidophoridae) cave crickets. J Mol Evol. 39:274–281.
- Betrán E, Rozas J, Navarro A, Barbadilla A. 1997. The estimation of the number and the length distribution of gene conversion tracts from population DNA sequence data. Genetics. 146:89–99.
- Charlesworth B, Sniegowski P, Stephan W. 1tt4. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature. 371:215–220.
- de Brito AR, Manfrin MH, Sene FM. 2002. Nested cladistic analysis of Brazilian populations of Drosophila serido. Mol Phylogenet Evol. 22: 131–143.
- Dorado O, Rieseberg LH, Arias D. 1992. Chloroplast DNA introgression in southern California sunflowers. Evolution. 46:566–572.
- Dover G. 1982. Molecular drive: a cohesive mode of species evolution. Nature. 299:111–117.
- Elder JF, Turner BJ. 1tt4. Concerted evolution at the population level: pupfish HindIII satellite DNA sequences. Proc Natl Acad Sci U S A. 91: 994–998.
- Excoffier L, Laval G, Schneider S. 2005. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online. 1:47–50.
- Excoffier L, Smouse PE, Quattro JM. 1992. Analyses or molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 131:479–491.
- Feliciello I, Picariello O, Chinali G. 2005. The first characterization of the overall variability of repetitive units in a species reveals unexpected features of satellite DNA. Gene. 349:153–164.
- Fernández Iriarte PJ, Norry FM, Hasson ER. 2003. Chromosomal inversions effect body size and shape in different breeding resources in Drosophila buzzatii. Heredity. 91:51–59.
- Franco FF, Kuhn GCS, Sene FM, Manfrin MH. 2006. Conservation of pBuM–2 satellite DNA sequences among geographically isolated Drosophila gouveai populations from Brazil. Genetica. 128:287–295.
- Franco FF, Prado PRR, Sene FM, Costa LF, Manfrin MH. 200G. Aedeagus morphology as a discriminant marker in two closely related cactophilic species of Drosophila (Diptera; Drosophilidae) in South America. An Acad Bras Cienc. 78:203–212.
- Franco FF, Sene FM, Manfrin MH. 2008. Molecular characterization of SSS13t satellite DNA family in sibling species of the Drosophila buzzatii cluster. Genet Mol Biol. 31:155–159.
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows t5/t8/NT. Nucleic Acids Symp Ser. 41:95–98.
- Hasson E, Fanara JJ, Rodríguez C, Vilardi JC, Reig OA, Fontdevila A. 1992. The evolutionary history of Drosophila buzzatii. XXIV. Second chromosome inversions have different average effect on thorax length. Heredity. 68:557–563.
- Hudson RR, Kaplan NL. 1985. Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics. 111:147–164.
- Kuhn GCS, Bollgönn S, Sperlich D, Bachmann L. 1999. Characterization of a species-specific satellite DNA of Drosophila buzzatii. J Zool Syst Evol Res. 37:109–112.
- Kuhn GCS, Franco FF, Manfrin MH, Moreira-Filho O, Sene FM. 2007. Low rates of homogenization of the DBC-150 satellite DNA family restricted to a single pair of microchromosomes in species from the Drosophila buzzatii cluster. Chromosome Res. 15:457–469.
- Kuhn GCS, Franco FF, Silva WA Jr, Martinez-Rossi NM, Sene FM. 2003. On the pBuM18t satellite DNA variability among South American populations of Drosophila buzzatii. Hereditas. 139:161–166.
- Kuhn GCS, Sene FM. 2004. Characterization and interpopulation variability of a complex HpaI satellite DNA of Drosophila seriema (repleta group). Genetica. 121:241–249.
- Kuhn GCS, Sene FM. 2005. Evolutionary turnover of two pBuM satellite DNA subfamilies in the Drosophila buzzatii species cluster (repleta group): from alpha to alpha/beta arrays. Gene. 349:77–85.
- Kuhn GCS, Sene FM, Moreira-Filho O, Schwarzacher T, Heslop-Harrison JS. 2008. Sequence analysis, chromosomal distribution and long-range organization show that rapid turnover of new and old pBuM satellite DNA repeats leads to different patterns of variation in seven species of the Drosophila buzzatii cluster. Chromosome Res. 16:307–324.
- Kumar S, Tamura K, Nei M. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 5:150–163.
- Lorite P, Carrillo JA, Garneria I, Petitpierre E, Palomeque T. 2002. Satellite DNA in the elm leaf beetle, Xanthogaleruca luteola (Coleoptera, Chrysomelidae): characterization, interpopulation analysis, and chromosome location. Cytogenet Genome Res. 98:302–307.
- Manfrin MH, Sene FM. 200G. Cactophilic Drosophila in South America: a model for evolutionary studies. Genetica. 126:57–75.
- Mateus RP, Sene FM. 2003. Temporal and spatial allozyme variation in the South American cactophilic Drosophila antonietae (Diptera; Drosophilidae). Biochem Genet. 41:219–233.
- Mateus RP, Sene FM. 2007. Population genetic study of allozyme variation in natural populations of Drosophila antonietae (Insecta, Diptera). J Zool Syst Evol Res. 45:136–143.
- Monteiro SG, Sene FM. 1tt5. Estudo morfométrico de populaxcões de Drosophila serido das regiões Central e Sul do Brasil. Rev Bras Genet (Suppl). 18:283.
- Moraes EM, Sene FM. 2007. Microsatellite and morphometric variation in Drosophila gouveai: the relative importance of historical and current factors in shaping the genetic population structure. J Zoolog Syst Evol Res. 45:336–344.
- Palomeque T, Lorite P. 2008. Satellite DNA in insects: a review. Heredity. 100:564–573.
- Pereira MAQR, Vilela CR, Sene FM. 1983. Notes of breeding and feeding sites of some species of the repleta group of the genus Drosophila (Diptera, Drosophilidae). Cienc Cult. 35:1313–1319.
- Picariello O, Feliciello I, Bellinero R, Chinali G. 2002. S1 satellite DNA as a taxonomic marker in brown frogs: molecular evidence that Rana graeca graeca and Rana graeca italica are diferent species. Genome. 45: 63–70.
- Ruiz A, Cassian AM, Kuhn GCS, Alves MAR, Sene FM. 2000. The Drosophila serido speciation puzzle: putting new pieces together. Genetica. 108:217–227.
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetics trees. Mol Biol Evol. 4:406–425.
- Sambrook J, Fritsh EF, Maniatis T. 1989. Molecular Cloning: a laboratory manual. 2nd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press.
- Smith GP. 1976. Evolution of repeated DNA sequences by unequal crossover. Science. 191:528–535.
- Strachan T, Webb D, Dover GA. 1985. Transition stages of molecular drive in multiple-copy DNA families in Drosophila. EMBO J. 4:1701–1708.
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680.
- Tidon-Sklorz R, Sene FM. 2001. Two new species of the Drosophila serido sibling set (Diptera, Drosophilidae). Iheringia. 90:141–146.
- Ugarkovic D, Plohl M. 2002. Variation in satellite DNA profiles—causes and effects. EMBO J. 21:5955–5959.
- Vilela CR. 1983. A revision of the Drosophila repleta species group (Diptera; Drosophilidae). Rev Bras Entomol. 27:1–114.
- Watabe H, Bachmann L, Haring E, Sperlich D. 1997. Taxonomic and molecular studies on Drosophila sinobscura and D. hubeiensis, two sibling species of the D. obscura group. J Zool Syst Evol Res. 35:81–94.
- Wu WL, Wang JP, Tseng MC, Chiang TY. 1999. Cloning and genetic variability of a HindIII repetitive DNA in Acrossocheillus paradoxos (Cyprinidae). Genome. 42:780–788.
To continue learning and get access to all the other articles, log in or create an account
